New treatment for symptoms of advanced Parkinson’s disease approved by FDA and unveiled at UK
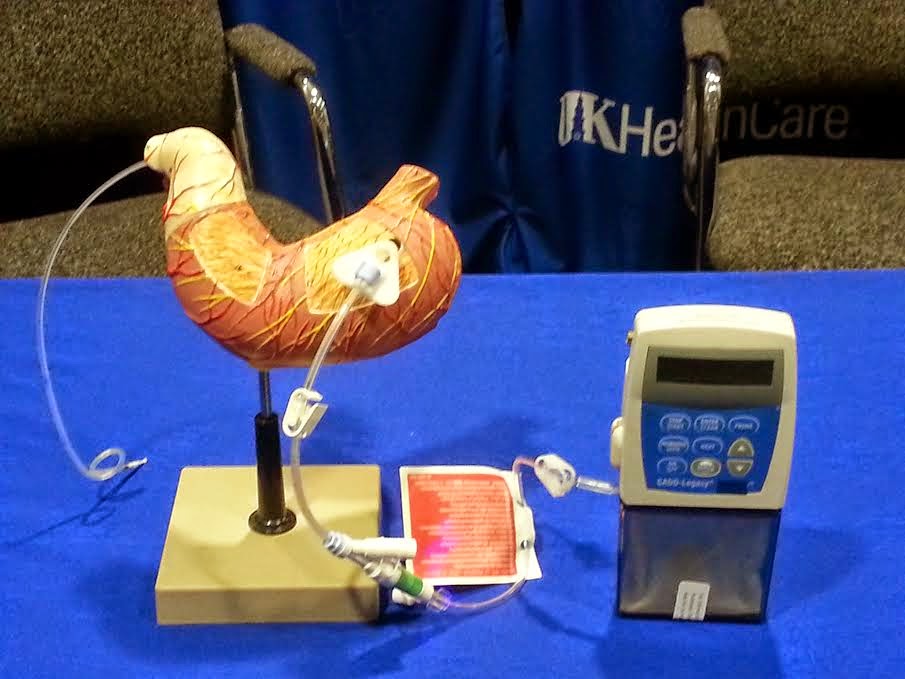
 |
| Portable infusion pump used to deliver Parkinson’s drug |
The University of Kentucky unveiled a new treatment for people with advanced symptoms of Parkinson’s disease at a news conference April 21 and invited one of the first patients to participate in the clinical trial to share how this treatment has improved his function and productivity.
The new, trademarked, treatment, Duopa, provides a continuous 16 hour dose of levodopa, which is the “gold standard” drug to treat Parkinson’s disease ,using a special gel preparation, is put directly into the small intestine by a portable infusion pump. It was developed by AbbVie Inc. and approved by the U.S. Food and Drug Administration in January.
“This treatment extends our ability to manage the signs and symptoms” of Parkinson’s, said Dr. John Slevin, professor of neurology and vice chair of research at UK’s Kentucky Neuroscience Institute. Slevin also worked with international investigators to test this treatment and is the lead author of the study, which is published in the Journal of Parkinson’s Disease.
Parkinson’s is a chronic and progressive disease of the nervous system that is characterized by motor symptoms such as tremors,slowness, stiffness and impaired balance and coordination. It can also cause non-motor symptoms such as sensory deficits, cognitive difficulties and sleep problems.
The cause of Parkinson’s is unknown and there is no cure, but it is known that the disease process involves the death of nerve cells in the brain that produce dopamine, a chemical that sends messages to the part of the brain that controls movement and coordination.
Slevin said that there are many challenges in treating the symptoms of Parkinson’s as it progresses. In addition to the continued loss of nerves in the brain, he said levodopa looses its effectiveness over time and the dose level begins to fluctuate. He also noted that over time patients will get a side-effect from the drug called dyskinesia, or involuntary muscle movement.
Another challenge, which he said prompted the development of this treatment, is that the muscles that control digestion are also affected by the disease, which creates an inconsistency in the blood level of levadopa that can be turned into dopamine in the brain.
This new treatment alleviates this challenge by placing the drug directly where it is absorbed in the intestine, allowing “The blood level, and thereby brain level (to remain) constant and that reduces the probability of having intermittent dyskinesia,” Slevin said.
 |
| Marion Cox |
“We are extremely pleased with the results,” Slevin said in a press release. “Patients with advanced Parkinson’s disease treated via this new method demonstrated marked improvement in symptom fluxuations and reduced dyskinesia.”
Marion Cox, a 70-year-old Georgetown farmer and former real estate developer, who has had Parkinson’s for 16 years, participated in the clinical trial for three years and said that this treatment has given him a “new lease on life.”
“It was the best thing that ever happened to me. The improvements have been that great,” Cox said. Later saying, “I can do anything I want to do. I can horseback ride. I’ve got a team of horses that I drive. I’ve got lots of farm equipment, excavating equipment that I drive. Before I went on the trial I was still doing those things, but unbelievably slow.”
 |
| Dr. Michael Karpf, Becky Cox, Marion Cox, Dr. John Slevin |
Dr. Michael Karpf, UK’s vice president for health, said he was proud to be part of a major health center committed to doing clinical trials: “What UK HealthCare has to do is to not (just) practice the standards of care, we have to move the standards of care forward.” Cox will be the first patient in Kentucky to receive Duopa after FDA approval.
Becky Cox, Marion’s wife of 25 years, said the treatment “saved him from an early retirement.” She noted that before Duopa, he had been taking an “unmanageable” number of pills to treat his symptoms, but now, after he hooks up to the pump in the morning, “It is a set it and forget it kind of thing. … He is off and running like he always used to be, so that has been a great blessing.”
Because this treatment involves an invasive procedure and because most people with Parkinson’s disease respond well to oral medication initially, Slevin said this treatment is meant for those with advanced Parkinson’s symptoms. He also said the cost for this treatment is still being determined, but it was already approved by Medicare. He noted that the next step will be to train other medical centers in how to deliver this treatment.
The National Parkinson Foundation website says 1 million people in the U.S. have the disease, with 50,000 and 60,000 new cases diagnosed each year. Kentucky has 14,000 people with it, Tony Bucalo, Parkinson’s neuroscience account executive at AbbVie, said after the news conference.