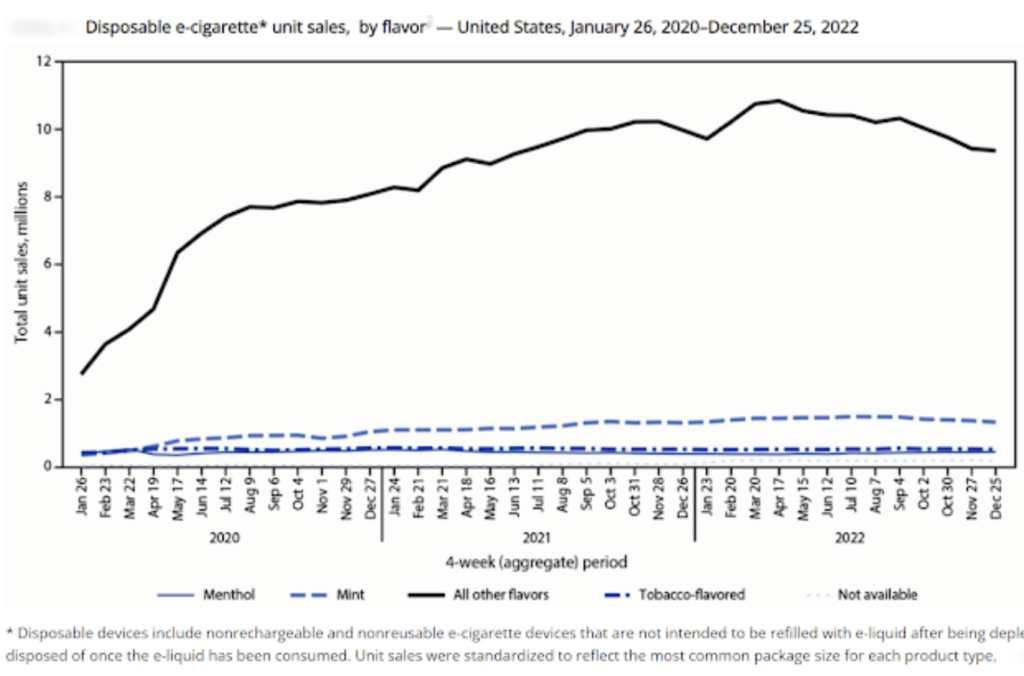
Instead, the FDA allowed all e-cigarettes already on the market to stay while their manufacturers applied for approvals to market them. Seven years later, vaping has ballooned into an $8.2 billion industry, and manufacturers are flooding the market with thousands of products — most sold illegally and without FDA permission — that can be far more addictive.
Sales of e-cigs have risen almost 47 percent in the last three years, according to the Centers for Disease Control and Prevention, and their nicotine content has gone up 76% over five years.
“The FDA has failed to protect public health,” said Eric Lindblom, former senior adviser in the FDA’s Center for Tobacco Products. “It’s a tragedy.”
The FDA isn’t the only entity that has tolerated the selling of vapes to children. Many players have declined to act, tied the agency’s hands, or neglected to provide the FDA with needed resources. Barack Obama and Donald Trump both prevented the FDA from broadly banning candy-flavored vapes.
Meanwhile, today’s vapes have become “bigger, badder, and cheaper” than older models, said Robin Koval, CEO of the Truth Initiative, a tobacco-control advocacy group. The enormous amount of nicotine in e-cigarettes can addict children in a matter of days, Koval said.
“We really don’t know the long-term health implications,” said Matthew Myers, president of the Campaign for Tobacco-Free Kids.
E-cigarettes in the U.S. now contain nicotine concentrations that are, on average, more than twice the level allowed in Canada and Europe. The U.S. sets no limits on the nicotine content of any tobacco product.
Elijah Stone of California was 19 when he tried his first e-cigarette at a party. He was a college freshman, grappling with depression and attention-deficit/hyperactivity disorder, and “looking for an escape.” Store clerks never asked for his ID. Stone said he was “hooked instantly.”
“The moment I felt that buzz, how was I supposed to go back after I felt that?” asked Stone, now 23.
The e-cigarette industry says higher nicotine concentrations can help adults who smoke heavily switch from combustible cigarettes to vaping products, which are relatively less harmful. The FDA has approved high-nicotine, tobacco-flavored e-cigarettes for that purpose, said April Meyers, CEO of the Smoke-Free Alternatives Trade Association.
“The goal is to get people away from combustible products,” said Nicholas Minas Alfaro, CEO of Puff Bar, one of the most popular brands with children last year. “These products are addictive products; there’s no hiding that.”
E-cigarettes don’t produce tar like combustibles, but contain harmful chemicals such as formaldehyde. The surgeon general has warned that vaping poses significant risks, including damage to the heart, lungs, and brain parts that control attention and learning, plus higher risk of addiction to other substances.
More than 2.5 million children used e-cigarettes in 2022, including 14% of high school students, according to the Centers for Disease Control. Most teen vapers in the U.S. begin puffing within an hour of waking up, according to a survey of e-cigarette users ages 16 to 19 presented at the Society for Research on Nicotine and Tobacco in March.
The potential for profits — and lax enforcement of vaping laws — has led to a gold rush. The number of unique vaping products, as measured by their bar codes, quadrupled in just one year, rising from 453 in June 2021 to 2,023 in June 2022, according to a Truth Initiative review of U.S. retail sales data.
FDA officials say they’ve been overwhelmed by the volume of e-cigarette marketing applications — 26 million in all.
“There is no regulatory agency in the world that has had to deal with a volume like that,” said Brian King, who became director of FDA’s Center for Tobacco Products in July 2022.
The agency has struggled to stop e-cigarette makers who continue selling vapes despite the FDA’s rejection of the products, as well as manufacturers who never bothered to apply for authorization, and counterfeiters hoping to earn as much money as possible before being shut down.
In 2018, public-health groups sued the agency, charging that the delay in reviewing applications put children at risk. A court ordered the FDA to finish the job by September 2021, but the FDA missed that deadline. An estimated 1.2 million people under the legal age of 21 began vaping in the next year, says a study published in May in the American Journal of Preventive Medicine.
The FDA recently said it has made decisions on 99% of applications, had rejected millions and authorized only 23. All authorized products have traditional tobacco flavors, and were deemed “appropriate for the protection of public health” because tobacco-flavored products aren’t popular with children but provide adult smokers with a less dangerous alternative, King said.
However, the agency has yet to make final decisions on the most popular products. Those applications are longer and need more careful scientific review, said Mitch Zeller, former director of the Center for Tobacco Products.
The FDA said it would not complete reviewing applications by the end of June, as it previously forecast, but would need until the end of the year.
Before the FDA can announce new tobacco policies, it needs approval from the president, and the White House doesn’t always agree with the FDA’s priorities.
Obama rejected FDA officials’ proposal to ban kid-friendly flavors in 2016, and in 2020, Trump backpedaled on his own plan to pull most flavored vapes off the market. Instead of banning all fruit and minty flavors, the administration banned them only in “cartridge-based” devices such as Juul. The flavor ban didn’t affect vapes without cartridges, such as disposable e-cigarettes.
The result was predictable, Zeller said. Teens switched in droves from Juul to brands that weren’t affected by the ban, including disposable vapes such as Puff Bar, which were allowed to continue selling candy-flavored vapes.
After receiving its own warning letter from the FDA last year, Puff Bar now sells only zero-nicotine vapes, Alfaro said.
When the FDA does attempt bold action, legal challenges often force it to halt or even reverse course.
The FDA ordered Juul to remove its products from the market in June 2022, but Juul sued and federal judges temporarily stayed the FDA’s order. Within weeks, the FDA announced it would hold off on enforcing the order because of “scientific issues unique to the Juul application that warrant additional review.”
E-cigarette makers Logic and R.J. Reynolds Vapor Co. both sued the FDA after the agency ordered them to stop selling menthol vapes, which are popular with teens. In both cases, court-imposed stays halted the FDA’s orders pending review and the companies’ menthol products remain on the market.
Under the Biden administration, the FDA has stepped up enforcement efforts. It fined 10 e-cigarette manufacturers more than $19,000 each, and issued more than 1,500 warning letters to manufacturers. It also issued warnings to 120,000 retailers for selling illegal products or selling to customers under 21, King said.
In October, the Justice Department for the first time filed lawsuits against six e-cigarette manufacturers on behalf of the FDA, seeking “to stop the illegal manufacture and sale of unauthorized vaping products.” In May, the FDA put Elfbar and other unauthorized vapes from China on its “red list,” which allows FDA agents to detain shipments without inspection at the border.
Some lawmakers say the Justice Department should play a larger role. “There are more than six e-cigarette manufacturers selling without authorization on the market,” Senate Majority Whip Dick Durbin of Illinois said in a March letter. Children are “vaping with unauthorized products that are on store shelves only because FDA has seemingly granted these illegal e-cigarettes a free pass.”
KFF Health News, formerly known as Kaiser Health News, is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at the Kaiser Family Foundation — the independent source for health-policy research, polling, and journalism.