Full House has Senate bill to regulate how druggists dispense ‘biosimilar’ medication that hasn’t even been approved by FDA
Brand-name drugs called “biologics,” because they are made from living tissues, have no competition from “interchangeable biosimilar” products in the U.S. because no interchangeable products have been approved by the U.S. Food and Drug Administration. But that hasn’t stopped the drug industry and Kentucky lawmakers from moving a bill to regulate how pharmacists could dispense these drugs if and when they are approved.
“This bill allows Kentucky pharmacists the ability to dispense safe and less expensive biological medications by allowing substitution of interchangeable biosimilars,” Sen. Ralph Alvarado, R-Winchester, told the House Health and Welfare committee, which approved it March 17.
Current law does not allow these substitutions without advanced approval from the prescriber, Alvarado said. “This bill removes that hurdle.”
The most contentious part of Senate Bill 134 has been its requirement that pharmacists must notify prescribers when they make this substitution, which advocates say is necessary because there are slight variations between the drugs.
Alvarado, also a physician, said the notification comes down to a “safety mandate,” noting that if the patient had a “bad outcome” while on one of these medications, it is important for the provider to know exactly what medication the patient is taking.
Democratic Rep. David Watkins, a retired physician from Henderson, voted for the bill and supported the provider notification requirement.
“We’re not talking about generics where you have exactly ideal medications, you are talking about biosimilars . . . which would be in some instances different molecules and have some different aspects,” Watkins said. ” I think that not notifying my office would be a gross disservice to my patients.”
But the pharmacists disagree, and want substitution of interchangeable biosimilars to be handled the same way as generic medications, with the prescriber able to place a note on the prescription that says “do not substitute,” said Bob Oakley, chairman of the Kentucky Pharmacists Association.
Oakley told the committee that while pharmacists support automatic substitution of an interchangable biosimilar for the name-brand biologic, they do not support notification. “Therefore, we are here to ask that we just keep it simple and keep it seamless,” he said.
Rep. Addia Wuchner, R-Florence, said the bill had accommodations to make notification manageable for pharmacists, and asked Oakley what their real problem was. He replied, “It just adds more work to . . . their busy day.”
But the pharmacists’ lobby has cited other reasons. KPhA Executive Director Bob McFalls told James McNair of the Kentucky Center for Investigative Reporting, “Prescriber notification requirements have shown to increase costs for the health-care system overall” since they cause more brand-name drugs to be dispensed.
SB 134 passed the Senate 36-1 March 2, with Republican Sen. Jimmy Higdon, the majority whip from Lebanon, the only one voting against it. Higdon had submitted several floor amendments to modify the notification requirements, but withdrew them before the final vote.
Higdon told McNair that he didn’t understand the urgency to pass this bill, noting that its passage would have no immediate effect.
“It’s the kind of bill that should be discussed,” Higdon said. “This whole thing is very complicated and futuristic, and a lot of people are talking to us about passing this. I just want to err on the side of caution. It doesn’t need to be fast-tracked. We need to do it right.”
Higdon was referring to the many lobbyists hired by pharmaceutical manufacturers that have descended on Kentucky in support of this bill.
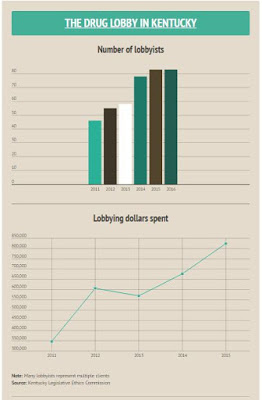
“At least nine drug companies and groups have stated an interest in the Senate bill, according to the Kentucky Legislative Ethics Commission, McNair reports. “The number of registered lobbyists hired by pharmaceuticals employers has nearly doubled, from 46 in 2011 to 83 today. Their annual spending has more than doubled, to $824,196 in 2015.”
The bill now resides in the House. Speaker Pro Tem Jody Richards, D-Bowling Green, has filed a floor amendment to remove the notification requirements.
About biologics and biosimilars
While conventional medications are made from pure chemical substances and can be easily replicated, biologics are made from living tissues and each batch varies slightly from the last, according to the FDA. That’s why these products can’t be called generics, which are chemcially identical.
The most common biologics are Humira and Remicade for arthritis and Enbrel for psoriasis. They are very expensive and can cost thousands of dollars each month.
“Express Scripts, the pharmacy benefits manager, estimates that while biologics accounted for only 1 percent of all prescriptions in 2014, they accounted for 32 percent of all prescription-drug spending,” McNair reports.
Biosimilars are medications that are “highly similar” to already FDA-approved biological products. To date, only one of these, Zarxio, used for certain cancer patients, has been approved by the FDA, but it was not designated as interchangeable. According to the Regulatory Affairs Professional Society, six biosimilars have applied for FDA approval.
Interchangeable biosimilars are expected to produce the same clinical results in any given patient as it’s “highly similar” biologic. To date, no interchangeable biosimilar medications have been approved by the FDA. When appoved, Alvarado suggested that they will cost up to 40 percent less than the biologics.
Kentucky is home to the North American distribution hubs for Amgen, Genentech and Johnson and Johnson and is the primary distribution point for many of the biologics (and soon to be biosimilars) in the U.S., according to an e-mail from RunSwitch PR.
